Have you ever wondered why ice doesn’t sink when you drop it into a glass of water? It’s a curious fact that goes against what you might expect—after all, solids usually sink in liquids.
But ice floats, and this simple phenomenon plays a huge role in our world, from keeping lakes alive in winter to shaping entire ecosystems. You’ll discover the surprising science behind why ice floats on water. Understanding this can change how you see everyday things and appreciate the hidden magic in something as ordinary as ice cubes in your drink.
Ready to uncover the mystery? Let’s dive in!
Water Molecules And Structure
Understanding why ice floats on water starts with exploring water molecules and their unique structure. Water’s behavior depends on how its molecules connect and move. This section breaks down these factors clearly.
Bent Shape Of Water Molecules
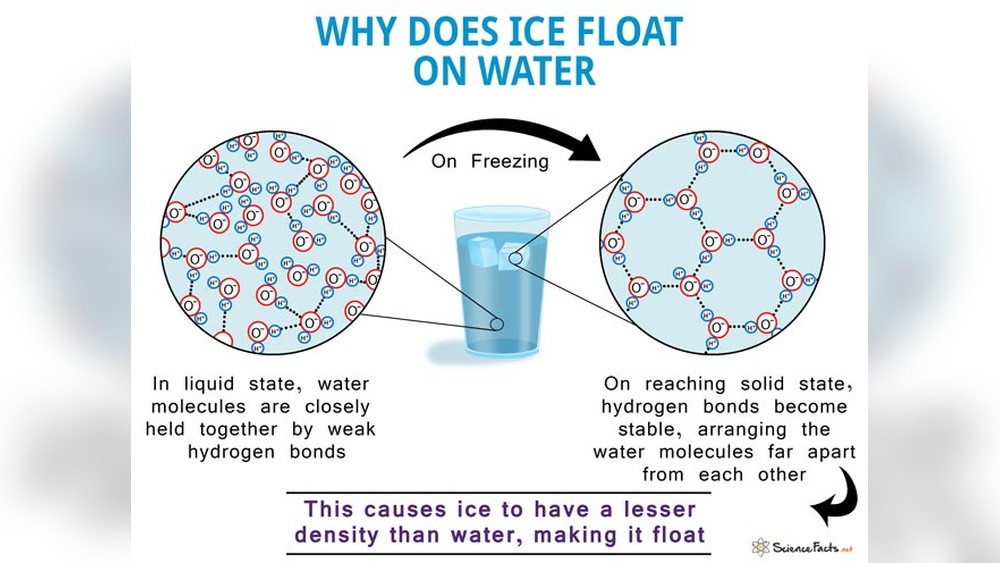
Water molecules have a bent or V shape. This shape is due to the oxygen atom bonding with two hydrogen atoms at an angle. The bent form creates a polar molecule with a positive and negative side. This polarity helps water molecules stick together strongly.
Hydrogen Bonding
Hydrogen bonds form between the positive hydrogen of one molecule and the negative oxygen of another. These bonds are weaker than regular chemical bonds but crucial for water’s properties. In ice, hydrogen bonds create a stable, open lattice structure that holds molecules farther apart. This spacing makes ice less dense than liquid water.
Molecular Movement In Liquid Water
In liquid water, molecules move freely and slide past each other. Hydrogen bonds break and reform quickly, allowing molecules to stay close. This close packing increases water’s density. When water cools and freezes, molecules slow down and lock into a rigid pattern, increasing space between them. This change causes ice to float.
Formation Of Ice
Ice forms through a fascinating process that changes water’s structure. This change affects its density and helps explain why ice floats. Understanding the formation of ice involves looking at how water molecules arrange themselves during freezing. The unique pattern they create causes ice to be lighter than liquid water.
Crystal Lattice Arrangement
Water molecules link together in a special pattern called a crystal lattice. Each molecule connects with four neighbors through hydrogen bonds. This creates a stable, repeating network. The lattice holds molecules apart, making ice less dense than liquid water.
Expansion During Freezing
As water cools, molecules slow down and arrange into the crystal lattice. This structure takes up more space than liquid water. The volume expands as water freezes. This expansion reduces the density of ice, allowing it to float on water’s surface.
Hexagonal Ice Structure
The crystal lattice of ice forms a hexagonal shape. This six-sided pattern is open and less packed. It traps more air and creates gaps between molecules. The hexagonal structure is key to ice’s lower density and its ability to float.
Density Differences
Density differences explain why ice floats on water. Density measures how much mass fits into a volume. Ice has a unique structure that changes its density compared to liquid water. This difference causes ice to be lighter and float above water.
Density Formula: Mass Vs Volume
Density is calculated by dividing mass by volume. The formula is simple: Density = Mass / Volume. If an object has the same mass but takes up more space, its density is lower. Objects with lower density float on those with higher density.
Why Ice Has Lower Density
Ice forms a crystal lattice when it freezes. This lattice creates open spaces between water molecules. These spaces increase ice’s volume without adding mass. More volume with the same mass means lower density. That is why ice is less dense than liquid water.
Comparison Between Ice And Liquid Water
Liquid water molecules move freely and stay close together. Ice molecules lock into a rigid, hexagonal pattern with gaps. Ice occupies more space than water for the same mass. This causes ice to float because it is less dense than the water below.

Credit: www.youtube.com
Floating Mechanism
The floating mechanism of ice on water is a fascinating natural phenomenon. It happens due to how ice and water differ in density and structure. Understanding this helps explain why ice does not sink but floats instead.
Buoyancy And Density Relationship
Objects float or sink based on their density compared to the liquid they are in. Density is mass divided by volume. If an object is less dense than the liquid, it floats. Ice is less dense than water. This means ice has less mass in the same amount of space.
Ice Floating In Water Explained
Water molecules form a special pattern when they freeze. They arrange in a hexagonal shape with gaps in between. These gaps make ice expand and become less dense. Liquid water molecules are closer together, so water is denser than ice. Because ice is lighter, it floats on water.
Examples In Nature
Ice floating helps lakes and rivers survive winter. A layer of ice forms on top, protecting the water and creatures beneath. Polar icebergs float on the ocean, showing this principle on a large scale. This floating ice supports ecosystems and affects climate.
Importance For Ecosystems
Ice floating on water plays a crucial role in maintaining healthy ecosystems. This unique property protects aquatic life during cold seasons and affects the environment around water bodies. Understanding its importance helps us appreciate how nature balances itself.
Insulation Of Aquatic Life
Ice forms a protective layer on the water surface. It keeps the water below warmer than the air above. Fish and other creatures survive under this ice during winter. Without floating ice, many species would struggle to live through freezing temperatures.
Prevention Of Frozen Water Bodies
Ice floating stops water bodies from freezing solid. The layer of ice insulates the liquid water beneath. This allows aquatic plants and animals to continue their life cycles. Frozen solid lakes would harm or kill many organisms that depend on liquid water.
Impact On Climate And Environment
Floating ice affects the Earth’s climate by reflecting sunlight. This helps regulate temperature in polar and temperate regions. Melting ice impacts weather patterns and ocean currents. Ice on water is vital for maintaining a stable environment worldwide.
:max_bytes(150000):strip_icc()/why-does-ice-float-604304_final-5c891ca046e0fb00010f1193.png)
Credit: www.thoughtco.com
Common Misconceptions
Many people have wrong ideas about why ice floats on water. These ideas come from what we usually see with other solids and liquids. Understanding these common mistakes helps us learn the unique behavior of water and ice.
Solids Usually Sink
Most solids are heavier than their liquid forms. For example, a rock sinks in water because it is denser. We expect ice to do the same. This is a natural thought but it is not true for water.
Unique Behavior Of Water
Water is special because it expands when it freezes. This is rare among liquids. Its molecules form a crystal structure that holds more space. This makes ice less dense than liquid water.
Clarifying Floating Phenomenon
Ice floats because it is lighter than the water it replaces. The open structure of ice traps air and spreads out molecules. This lowers its density, allowing it to float on water.

Credit: www.nagwa.com
Frequently Asked Questions
What Why Does Ice Float On Water?
Ice floats on water because its molecules expand and form a lattice structure when frozen. This makes ice less dense than liquid water, causing it to float.
Why Is Ice Less Dense Than Water?
Ice is less dense than water because its molecules form a hexagonal lattice with large gaps. This structure increases ice’s volume, reducing its density. Water molecules in liquid form stay closer together, making water denser than ice. This allows ice to float on water.
Why Does Ice Float In Water A Level In Chemistry?
Ice floats on water because its molecules form an open lattice when frozen, increasing volume and decreasing density. This makes ice less dense than liquid water, allowing it to float.
What Does It Mean If Ice Cubes Sink?
Ice cubes sink when they are denser than the liquid, often due to impurities or salt in the ice or water.
Conclusion
Ice floats on water because it is less dense. Water molecules spread out when frozen, forming a crystal structure. This makes ice take up more space than liquid water. Since ice weighs less for the same volume, it stays on top.
This simple fact helps protect fish and plants in cold weather. Understanding why ice floats shows how nature works in surprising ways. It also explains many natural phenomena we see in daily life. Keep exploring science; it reveals the world around us.